วิทยาศาสตร์ชีวภาพ
เครื่องมือสำหรับ
Lab-On-Chip (LOC)
Veredus Laboratories ผลิตและจำหน่ายชุดตรวจจับ VerePLEX™ Biosystem และ LOC panel ของชุดตรวจจับเพื่อให้การขยาย PCR แบบมัลติเพล็กซ์พร้อมการตรวจหา microarray สำหรับการใช้งานในการเฝ้าระวังทางชีวภาพ (Bio surveillance), Biological Threat detection, การตรวจจับเชื้อโรคจากมนุษย์ อาหารและน้ำ รวมทั้งการทดสอบที่กำหนดเองได้
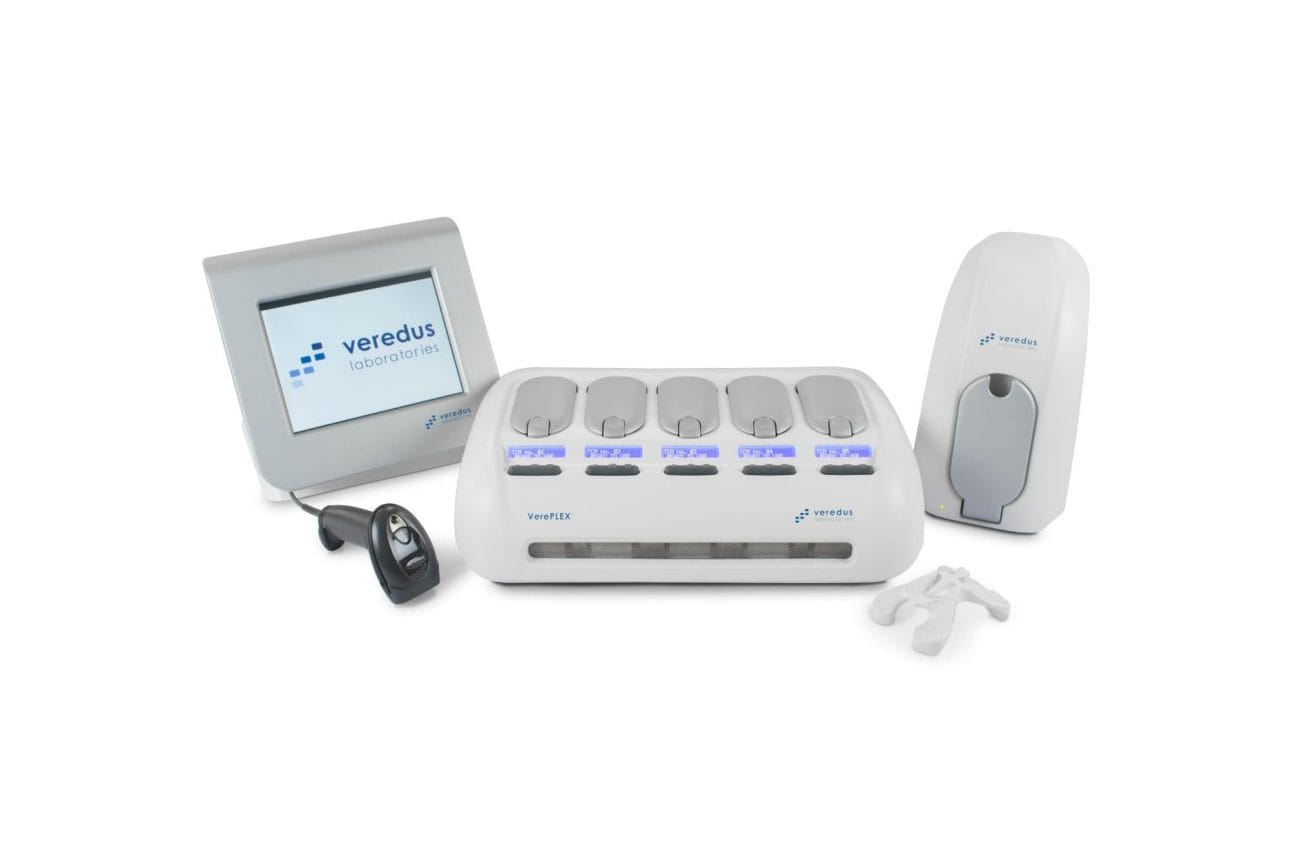

Veredus Laboratories เพิ่มขีดความสามารถในการทดสอบที่เป็นนวัตกรรมใหม่ด้วยการเข้าถึงแบบสุ่มสำหรับการทดสอบตามความต้องการเมื่อห้องปฏิบัติการจำเป็นต้องระบุ Source Agent อย่างรวดเร็วในสถานการณ์ที่ไม่ทราบสาเหตุ เช่นการระบาดทางชีวภาพ หรือการระบาดของอาหารเป็นพิษ ซึ่งใช้ปริมาณตัวอย่างเพียงเล็กน้อยสำหรับการตรวจจับที่ละเอียดอ่อน และการระบุกรดนิวคลีอิกและToxin Genes ของเชื้อโรคและ biothreat agent หลายชนิดภายในการทดสอบเดียวกัน
กรุณาคลิกปุ่มด้านล่างเพื่อดูข้อมูลเพิ่มเติมเกี่ยวกับการตรวจทางอณูชีววิทยา(Lab-on-Chip)
การตรวจทางอณู
ชีววิทยาโดยใช้ LOC
LOC Panel ของ Veredus ประกอบด้วย 3 ส่วนใหญ่ๆ ส่วนหลักคือ Biosurveillance ความปลอดภัยของอาหารและโรคติดเชื้อ ปัจจุบัน Panel ได้แก่ VereBeef™, VereFoodborne™, VereFever™, VereFluPlus™, VereMTB™, VereThreat™, VereMERS™, VereCoV™


COVID-19 Resources
ในการระบาดใหญ่ของ COVID-19 นี้ Veredus มีผลิตภัณฑ์เพื่อสนับสนุนและตอบสนองความต้องการในการทดสอบทางคลินิก เรามีการวินิจฉัยที่ครอบคลุม เริ่มตั้งแต่การสกัด RNA จากไวรัสไปจนถึงการตรวจเชื้อวัณโรคดื้อยาด้วยอณูชีววิทยาเพื่อตรวจหา SARS-CoV-2 นอกจากนี้เรายังมีการตรวจจับอย่างรวดเร็วโดยใช้ Serological testing อีกด้วย
VereBeef™ Detection Kit is a multiplex test combining both Polymerase Chain Reaction (PCR) and microarray technologies into a single testing workflow. This product is intended for qualitative detection, differentiation and identification of multiple food-borne pathogens in raw beef trim and is certified for use by the Association of Official Analytical Chemists (AOAC Research Institute) in the US.
This test is for use in conjunction with the VerePLEX™ Biosystem.
One or more of the following targeted organisms can be identified with VereBeefTM Detection Kit:
• Escherichia. coli O157:H7
• non-O157 Shiga toxin-producing E. coli (STEC) serogroups
• O26
• O45
• O103
• O111
• O121
• O145
• Salmonella species
The virulence genes targeted for E.coli analysis are:
• eae (intimin)
• stx (Shiga-like toxins)
VereFoodborne™ Detection Kit is a multiplex test combining both Polymerase Chain Reaction (PCR) and microarray technologies into a single testing workflow. This product is intended for qualitative detection, differentiation and identification of major food pathogens in a single test. This is a Research Use Only (RUO) test.
This test is for use in conjunction with the VerePLEX™ Biosystem.
One or more of the following targeted organisms can be identified with VereFoodborne™ Detection Kit:
• Bacillus species
• Campylobacter jejuni / lari / coli
• Clostridium perfringens
• Cronobacter sakazakii
• Listeria species
• Salmonella species
• Shiga toxin-producing Escherichia Coli (STEC)
• Shigella species
• Staphylococcus aureus
• Vibrio cholera
• Vibrio parahaemolyticus
• Shiga toxin genes (stx1A, stx2A)
VereFever™ Detection Kit is a multiplex test combining both Polymerase Chain Reaction (PCR) and microarray technologies into a single testing workflow. This product is intended for qualitative detection, differentiation and identification of multiple vector-borne pathogens in a single test. This is a Research Use Only (RUO) test.
This test is for use in conjunction with the VerePLEX™ Biosystem.
One or more of the following targeted organisms can be identified with VereFever™ Detection Kit:
• Malaria parasites
• Plasmodium falciparum
• Plasmodium vivax
• Plasmodium malariae
• Plasmodium ovale
• Plasmodium knowlesi
• Chikungunya virus
• Dengue virus serotypes (1-4)
• Japanese Encephalitis virus
• West Nile virus
• Yellow Fever virus
• Zika virus
VereFluPlus™ Detection Kit is a multiplex test combining both Polymerase Chain Reaction (PCR) and microarray technologies into a single testing workflow. This product is intended for the qualitative detection, differentiation and identification of Influenza A and/or Influenza B virus in a single test. This is a Research Use Only (RUO) test.
This test is for use in conjunction with the VerePLEX™ Biosystem.
Based on a test matrix concept, one or more of the following targets can be identified with VereFluPlus™ Detection Kit:
• Influenza A
• Influenza B
• H1
• H3
• H5
• H7
• H9
• N1
• N2
• N6
• N8
• N9
VereMTB™ Detection Kit is a multiplex test combining both Polymerase Chain Reaction (PCR) and microarray technologies into a single testing workflow. This product is intended for qualitative detection, differentiation and identification of multiple pathogens in a single test. This is a Research Use Only (RUO) test.
This test is for use in conjunction with the VerePLEX™ Biosystem.
One or more of the following targets can be identified with VereMTB™ Detection Kit:
• Mycobacterium tuberculosis complex (MTBC)
• Mycobacterium tuberculosis (MTB) resistance or sensitivity to
• Rifampicin
• Isoniazid
The test also detects and differentiates 14 nontuberculous mycobacteria (NTM) consisting of
• M. abscessus, M. chelonae
• M. avium
• M. intracellulare, M. vulneris, M.chimaera, M.colombiense
• M. simiae, M. kansasii, M. scrofulaceum, M. gastri, M. mantenii
• M. xenopi
• M. fortuitum
VereThreat™ Detection Kit is a multiplex test combining both Polymerase Chain Reaction (PCR) and microarray technologies into a single testing workflow. This product is intended for qualitative detection, differentiation and identification of biological agents in a single test. This is a Research Use Only (RUO) test.
This test is for use in conjunction with the VerePLEX™ Biosystem.
One or more of the following targets can be identified with VereThreat™ Detection Kit:
• Bacillus anthracis
• Francisella tularensis
• Yersinia pestis
• Variola virus
VereMERS™ Detection Kit is a multiplex test combining both Polymerase Chain Reaction (PCR) and microarray technologies into a single testing workflow. This product is intended for the qualitative detection and identification of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in a single test. This is a Research Use Only (RUO) test.
This test is for use in conjunction with the VerePLEX™ Biosystem
VereCoV™ OneMix Detection Kit is a multiplex Reverse Transcription Polymerase Chain Reaction (RT-PCR)-based In Vitro Diagnostic (IVD) COVID-19 test intended for the qualitative detection of nucleic acid belonging to SARS-CoV-2. This test is for use in conjunction with VerePLEX™ Biosystem and the test results can be used as supplementary data for diagnosis. Negative result does not preclude SARS-CoV-2 infection and should not be used as a sole basis for treatment or other patient management decisions. Testing with VereCoV™ OneMix Detection Kit is intended for use by trained laboratory professionals who are proficient in using the VerePLEX™ Biosystem.
VereCoV™ OneMix Detection Kit is an improvement to VereCoV™ Detection Kit which is under Provisional Authorisation for IVD use issued by Health Sciences Authority (HSA) Singapore. This kit is also CE-IVD marked.
Compatibility
• CommaXP® Virus DNA / RNA Extraction Kit
• QIAamp® Viral RNA Mini Kit
Gene Targets
• ORF1ab
• Spike (S)
• Nucleocapsid (N)
Sensitivity
• 20 viral RNA copies per test
Turnaround Time
• Approximately 120 mins
VereRT™ COVID-19 PCR Kit is a one-step Reverse Transcription Polymerase Chain Reaction (RT-PCR)-based In Vitro Diagnostic (IVD) COVID-19 test intended for the qualitative detection of nucleic acid belonging to SARS-CoV-2. This test is suitable for use with extracted viral RNA from nasopharyngeal swab specimen in transport media and the test results can be used as supplementary data for diagnosis. Negative result does not preclude SARS-CoV-2 infection and should not be used as a sole basis for treatment or other patient management decisions. Testing with VereRT™ COVID-19 PCR Kit is intended for use by trained laboratory professionals who are proficient in performing real-time RT-PCR assays.
VereRT™ COVID-19 PCR Kit is under Provisional Authorisation for IVD use issued by Health Sciences Authority (HSA) Singapore. Veredus is authorised to supply VereRT™ COVID-19 PCR Kit to the healthcare institutions, private hospitals, medical clinics or clinics laboratories under the PHMC Act (Cap. 248) for use on their patients. This kit is also CE-IVD marked and approved by Philippines FDA for IVD use.
Veredus is authorised to export VereRT™ COVID-19 PCR Kit out of Singapore subject to the duties and obligations as stipulated in the Health Products Act and the Health Products (Medical Devices) Regulation 2010.
Compatibility
• CommaXP® Virus DNA / RNA Extraction Kit
• QIAamp® Viral RNA Mini Kit
Gene Targets
• Dual N gene targets (FAM)
• Human RPP30 (HEX)
Sensitivity
• 2 viral RNA copies per test
Turnaround Time
• Approximately 90 mins
VereRT™ ZeroPrep™ COVID-19 PCR Kit is a one-step Reverse Transcription Polymerase Chain Reaction (RT-PCR)-based In Vitro Diagnostic (IVD) COVID-19 test intended for the qualitative detection of nucleic acid belonging to SARS-CoV-2.
This test is suitable for use directly from either (1) Viral Transport Media (VTM) / Universal Transport Media (UTM) containing nasopharyngeal swab specimen; OR (2) Human saliva specimen. Both specimen types do not require viral RNA extraction prior to testing and the test results can be used as supplementary data for diagnosis. Negative result does not preclude SARS-CoV-2 infection and should not be used as a sole basis for treatment or other patient management decisions. Testing with VereRT™ ZeroPrep™ COVID-19 PCR Kit is intended for use by trained laboratory professionals who are proficient in performing real-time RT-PCR assays.
VereRT™ ZeroPrep™ COVID-19 PCR Kit is under Provisional Authorisation for IVD use issued by Health Sciences Authority (HSA) Singapore. Veredus is authorised to supply VereRT™ ZeroPrep™ COVID-19 PCR Kit to the healthcare institutions, private hospitals, medical clinics or clinics laboratories under the PHMC Act (Cap. 248) for use on their patients. This kit is also CE-IVD marked.
Veredus is authorised to export VereRT™ ZeroPrep™ COVID-19 PCR Kit out of Singapore subject to the duties and obligations as stipulated in the Health Products Act and the Health Products (Medical Devices) Regulation 2010.
Compatibility
• VTM* / UTM* containing nasopharyngeal swab specimen
• Human saliva specimen collected using ZeroPrep™ Saliva Collection Kit
* validated with 5 different brands of VTM /UTM
Gene Targets
• Dual N gene targets (FAM)
• Human RPP30 (HEX)
Sensitivity
• 10 viral RNA copies per test
Turnaround Time
• Approximately 90 mins
ZeroPrep™ Saliva Collection Kit is a specimen device intended for the collection of human saliva with the preservation buffer designed to protect and preserve viral RNA suitable for use directly in genetic and molecular diagnostic testing. This kit is compatible for use together with the VereRT™ ZeroPrep™ COVID-19 PCR Kit for the diagnosis of COVID-19.
ZeroPrep™ Saliva Collection Kit is registered as a Class A Medical Device with the Health Sciences Authority (HSA) Singapore.
Veredus is authorised to export ZeroPrep™ Saliva Collection Kit out of Singapore subject to the duties and obligations as stipulated in the Health Products Act and the Health Products (Medical Devices) Regulation 2010.
Compatibility
• VereRT™ ZeroPrep™ COVID-19 PCR Kit
บริษัท เซกิซุย เซ้าท์อีสท์ เอเชีย จำกัด
Unit 9B,9C,9L,9M, 9th Floor, President Tower
973 ถนนเพลินจิต
เขตปทุมวัน กรุงเทพมหานคร 10330
ประเทศไทย